New Genetic Form of Pulmonary Alveolar Proteinosis (PAP), Diagnostic Tests, and Effective Potential Treatment Identified
Pulmonary alveolar proteinosis (PAP) was discovered in 1958 but it wasn’t until almost 40 years later that the pathogenesis of this rare lung syndrome was determined following the serendipitous discovery that mice deficient in granulocyte-macrophage colony-stimulating factor (GM-CSF) spontaneously develop PAP. Studies at the University of Cincinnati Medical Center demonstrated that GM-CSF, a small signaling protein, was necessary for the maturation of alveolar macrophages and to enable clearance of surfactant and other substances from the lungs. Without GM-CSF signaling, macrophages did not mature properly and are not able to remove excess surfactant, resulting in surfactant accumulation in alveoli and development of PAP. These findings paved the way for further advances for patients with PAP.
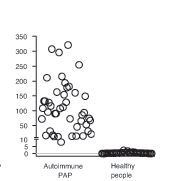
Serum GM-CSF autoantibody measurement using an enzyme-linked immunosorbent assay (ELISA) provides a simple, blood-based method to diagnose autoimmune PAP. The assay, which is based on an ELISA initially established in Japan by Professor Koh Nakata, is the only method available to specifically identify autoimmune PAP and is highly sensitive and specific for the diagnosis. Adapted from Uchida, et al.1 Healthy controls are clustered near the lower limit of detection.
Following a subsequent observation that GM-CSF autoantibodies were associated with PAP, researchers at the University of Cincinnati Medical Center led by Bruce Trapnell, MD, Professor of Medicine and Pediatrics, demonstrated that GM-CSF autoantibodies caused the most common PAP-associated disease (autoimmune PAP) and established a panel of biomarker-based blood tests for its clinical research diagnosis (Figure).
Clinical research utilizing this test identified a child with biopsy-proven PAP who was negative for GM-CSF autoantibodies. The evaluation led to identification of hereditary PAP (hPAP), a new genetic form of PAP caused by GM-CSF receptor gene mutations, in addition to the development of additional biomarker-based blood tests useful for the differential diagnosis of PAP that are capable of identifying the PAP-causing disease in more than 90% of patients.
In considering potential therapeutic approaches and prompted by recent basic science research results documenting that resident macrophage populations self-maintain independently of hematological progenitors, Trapnell’s team investigated pulmonary macrophage transplantation as a novel organ-targeted, cell-specific treatment approach for hPAP,2 with results recently published in Nature. “In mice with GM-CSF receptor defects – that develop hPAP identical to the disease in children, after a single transplantation of normal or gene-corrected macrophages into the lungs, the transplanted cells survive, replicate, mature normally, and replace the endogenous alveolar macrophage population over time, such that after a year, the disease is improved by more than 90% and all the disease biomarkers are reset,” Trapnell says. His team received funding from the National Institutes of Health (NIH) to conduct pre-clinical work and hopes to begin human trials in two to three years. This study is one of several treatment trials to be conducted by researchers at the University of Cincinnati in conjunction with the Rare Lung Diseases Consortium.
Two decades of translational PAP research in mice and man have not only identified the critical role of GM-CSF in the lung and provided a robust understanding of the role of GM-CSF in immunity and lung disease, but have also turned the pathogenic driver of a rare lung disease – GM-CSF antibodies in autoimmune PAP – into a novel treatment approach for common diseases such as rheumatoid arthritis and severe neutrophilic asthma.
Patients and clinicians already benefit from the establishment of the blood tests for PAP that are available in the US only through the Translational Pulmonary Science Center (TPSC) at the University of Cincinnati Medical Center3. Trapnell is spearheading efforts to bring them into routine clinical use. These tests exemplify the central goal of TPSC – to translate basic and clinical research findings into the clinic to benefit patients in real time.
References:
1. Uchida K, Nakata K, Carey B, Chalk C, Suzuki T, Sakagami T, et al. Standardized serum GM-CSF autoantibody testing for the routine diagnosis of autoimmune pulmonary alveolar proteinosis. J Immunol Methods, 2014; 402:57-70.
2. Suzuki T, Arumugam P, Sakagami T, Lachmann N, Chalk C, Sallese A et al. Pulmonary macrophage transplantation therapy. Nature, 2014; 514:450-454.
3. med.uc.edu/tpsc
 Bruce Trapnell, MS, MD
Bruce Trapnell, MS, MD
Professor of Medicine and Pediatrics
University of Cincinnati Medical Center
Medical School: University of Maryland
(513) 636-6956
Bruce.Trapnell@cchmc.org

