Case Study Update: Novel Use of Intra-Bronchial Valves (IBVs) in Patient with Chronic, Refractory Bronchopleural Fistula Extends the Utility of the Technique
Intra-bronchial valves (IBVs) are FDA-approved to limit distal airflow to damaged lung tissue to aid in the reduction or cessation of persistent postoperative air leaks in pre-identified airways.1 However, the technique has been utilized successfully in other cases as well. As reported in the February 2014 issue of the American Journal of Respiratory and Critical Care Medicine, physicians at the University of Cincinnati Medical Center evaluated a 39-year-old nonsmoking female patient with the rare disease, lymphangioleiomyomatosis. She presented with a right-side pneumothorax and persistent bronchopleural fistula that had failed to resolve after multiple prior chest tube drainage procedures, and thoracic surgical interventions including mechanical pleurodesis, bleb resection, and pleurectomy.2
The team initiated treatment with one-way IBVs, which succeeded in reducing the rate of air leakage to levels that allowed for pleural fusion upon pleurodesis with talc.2 The endobronchial valves were removed without complications, and at a follow-up visit three months later, the patient remained asymptomatic, with normal pulmonary function tests and a CT scan of the chest showing complete resolution of the pneumothorax.2 “In this patient’s case, we used the valves for a novel purpose, to seal a chronic air leak in order to obtain good apposition of the lung and chest wall and optimize our chances of effective pleurodesis,” said Sadia Benzaquen, MD, University of Cincinnati Medical Center Director of Interventional Pulmonology. The patient returned to work and maintains a consistent exercise routine. She is being treated with sirolimus to prevent progression of LAM3. This case illustrates a novel, off-label use of existing technology to treat a chronic pneumothorax, and demonstrates that IBVs may have applications beyond their currently-approved use. Benzaquen is the Site PI for the Evaluation of the IBV® Valve for Emphysema to Improve Lung Function (EMPROVE) trial.
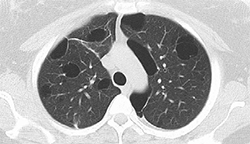
 1. Pre-IBV placement showing upper lobe cysts |
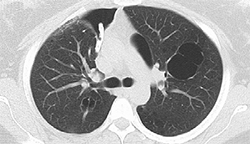 2. Pre-IBV valve placement showing CT on a right-sided pneumothorax |
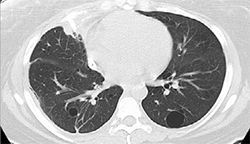 3. Right-sided CT with IBV valves in place |
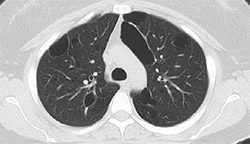 4. Pneumothorax resolved after IBV and talc pleurodesis |
 Sadia Benzaquen, MD
Sadia Benzaquen, MD
Assistant Professor, Department of Internal Medicine
Director of Interventional Pulmonology
University of Cincinnati Medical Center
Medical School: Luis Razetti School of Medicine, Universidad Central de Venezuela, Caracas, Venezuela
(513) 475-8523
benzaqsa@ucmail.uc.edu

