Technological Advances and Research Continue to Transform Hearing Outcomes
Digital technology has grown exponentially since its introduction for hearing aids around 1995, says Stephanie Lockhart, MA, audiology director, University of Cincinnati (UC) Medical Center. As UC’s audiology program (as it is not its own department) helps patients keep current with advances in hearing devices, research is also contributing to improved hearing for patients, such as a new Hybrid-L hearing device trial that may offer an option for patients with few therapeutic options.
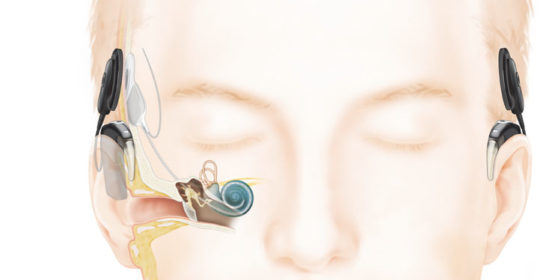
As hearing loss progresses and patients stop receiving a significant benefit from hearing aids a cochlear implant may be suggested. Cochlear implants have the ability to not only provide audibility of sounds but are also about to provide clarity over time as they are replacing the part of the ear that is damaged. Cochlear implants today are now able to take advantage of “true wireless” connectivity as well. Image courtesy of Cochlear Americas.
According to Lisa Houston, AuD CCC-A, F-AAA, head cochlear implant audiologist/clinical audiologist, three UC Medical Center patients were part of the initial “Evaluation of the Nucleus Hybrid L24 Cochlear Implant System” trial,1 and one was a member of the panel that presented the case for approval for the cochlear Hybrid-L to the Food and Drug Administration (FDA). (The trial was conducted from 2008 to 2014; the FDA approved the Hybrid-L device in 2014.2) Dr. Houston describes how the Hybrid device functions: the combination of electric stimulation and acoustic amplification in one device greatly improves the hearing of persons with significant high frequency sensorineural hearing loss, while preserving normal anatomic structure. The implanted components are a cochlear receiver/stimulator assembly with a Hybrid electrode array (which is a shortened electrode array); the external components comprise a sound processor with an acoustic component and accessories.3 Trial investigators found that most people reported significant improvement after six months in their ability to recognize words and sentences compared with their understanding when using a hearing aid before implantation.3 UC Medical Center continues to collect data for the FDA on the effectiveness of the device.
In a second research effort, “UC Medical Center is a test site for the audiology assessment arm of the Conservation of HEARing (CHEARS) study,” Lockhart says. This trial is assessing the epidemiology of acquired hearing loss, in particular, potentially modifiable risk factors (i.e., medical, dietary, and lifestyle), and hearing loss.4 Data is being gathered through hearing study questionnaires and from audiologic assessments. Most of the CHEARS’ work is based on data collected from three major ongoing cohort studies of 250,000 participants that comprise the Nurses’ Health Study.
Hearing-impaired patients at UC Medical Center are introduced to the latest technology that suits their needs. “New technology arrives every 1½ to 2 years with the six major manufacturers introducing new features such as a computer chip or a new platform,” says Lockhart. The new technology – wireless streaming, iPhone connectivity, external devices (e.g., external microphones, phone-clip accessories) – is adapted to cochlear implants, as well as hearing aids. UC Medical Center patients who are using an iPhone with the GN ReSound Smart Hearing aid, Linx, are pleased with their improved hearing. Lockhart says that “older patients, in their 70s and 80s, are enthusiastic about using iPhone hearing aids. It’s greatly improved their quality of life.” ReSound and Cochlear Americas have partnered to share iPhone compatibility and wireless streaming technology so that a patient can have the same wireless benefits with their hearing aids and cochlear implants.

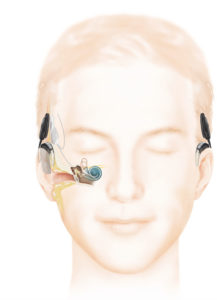
The Resound Linx hearing aid communicates via Bluetooth directly with IPhone, IPad, and IPod and will communicate with other wireless devices using 2.4 GHz wireless technology. Pictured is the Receiver-In-Canal model. Image courtesy of ReSound North America.
Lockhart, whose clinical focus is hearing aids, says these are “the most common treatment for sensorineural hearing. When they no longer provide effective amplification, a specialist [like Dr. Houston] takes over.” Dr. Houston specializes in cochlear implants and other implantable devices, and describes the patient population for these devices as people “who no longer benefit from traditional hearing aids and can be helped by a cochlear implant or osseointegrated hearing implant].” In 2015, Dr. Houston and her colleagues saw 442 patients for cochlear implants and follow-up visits. “While cochlear implants were considered a last resort years ago, they are no longer only for profoundly hearing impaired people. Now, even people with good word understanding and moderate to profound hearing loss can benefit.” The chief advantage of new devices and technology, Dr. Houston says, is that “patients feel more comfortable because everyone else is using similar technology.”
References:
- Evaluation of the Nucleus HybridTM L24 cochlear implant system. Available at https://clinicaltrials.gov/ct2/show/NCT00678899?term=cochlear+hybrid+L24&rank=1. Accessed March 22, 2016.
- FDA approves first implantable hearing device for adults with a certain kind of hearing loss. March 20, 2014. US Department of Health and Human Services. US Food and Drug Administration. Available at http://www.fda.gov/newsevents/newsroom/pressannouncements/ucm389860.htm. Accessed March 21, 2015.
- Cochlear Nucleus Hybrid L24 cochlear implant. Package insert. Cochlear Ltd. New South Wales, Australia. 2014. Available at http://www.cochlear.com/wps/wcm/connect/shared-library/downloads/cam-downloads/indications/hybrid-indications. Accessed March 22, 2016.
- CHEARS: Conservation of Hearing Study. Available at http://www.chearsstudy.org. Accessed March 20, 2016.
 Lisa Houston, AuD CCC-A, F-AAA
Lisa Houston, AuD CCC-A, F-AAA
Cochlear Implant Audiologist/Clinical Audiologist
UC Health Department of Otolaryngology Head and Neck Surgery
513-475-8453
Lisa.Houston@UCHealth.com
 Stephanie Lockhart, MA
Stephanie Lockhart, MA
Audiology Director
UC Health Audiology & Hearing Aids
(513) 475-8453
Stephanie.Lockhart@UCHealth.com

