Facial Muscle Cells May Help Repair Damage from Cardiovascular Disease
Researchers at the University of Cincinnati Medical Center (UC Medical Center) are investigating the use of facial muscle-derived progenitor cells – masseter muscle cells – to regenerate cardiomyocytes damaged by cardiovascular disease. Current therapies, such as medications, bypass surgery and donor hearts do not repair or replace cardiomyocytes and come with additional risks such as surgical complications and rejection, among others. The research, directed by Yigang Wang, MD, PhD, professor of medicine and director of the regenerative medicine division, at University of Cincinnati College of Medicine, is funded by a $2.4 million National Institutes of Health Research Project Grant.
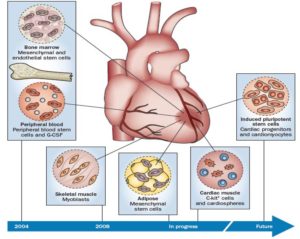
Milestones in clinical trials of cardiac regenerative therapies. Reprinted with permission from Macmillan Publishers Ltd: Nature Reviews Cardiology, Fuster V. Top 10 cardiovascular therapies and interventions for the next decade. Nat Rev Cardiol. 2014, 11(11): 671, copyright 2014.
Since 2006, researchers in the field of cardiac regenerative medicine have focused on finding a method of replacing cardiomyocytes that are lost through myocardial infarction. Induced pluripotent stem cells are potentially a good cell source because they can generate a large number of functional cardiovascular cells, explained Dr. Wang. However, progenitor cells derived from masseter muscle are related to stem cells of the second heart field.1 They show overlapping patterns of gene expression, which may result from a shared embryonic origin for both myocardial and masseter muscle progenitor (MMP) cells, indicating that MMP cells could be used to generate cardiogenic lineages, thus providing a significant source of autologous cardiomyocytes.2 As Dr. Wang explained, “obtaining progenitor cells from a patient’s own masseter muscles can avoid common problems, such as tumor formation and immunorejection that are caused by other cell based therapies after myocardial infarction.”
In small animal studies, data yet to be published, Dr. Wang and his colleagues have demonstrated the cardiac nature of MMP cells. These progenitor cells possess cardiogenic potential, as indicated by specific cardiogenic gene expression, morphology and electrophysiological characteristics. The UC Medical Center team is also studying microRNAs to gain a better understanding of MMP-derived cardiomyocyte differentiation. Wei Huang, PhD, a research associate in the Department of Pathology and Laboratory Medicine, described how blocking the activity of a specific microRNA (miR128) affected the heart’s self-healing ability. Mice with elevated levels of miR128 had reduced cardiomyocyte growth; however, mice with miR128 deletion had increased cardiomyocyte growth and recovered better after heart attacks. These results show that miR128 controls the generation of cardiomyocytes and may be a promising therapy for heart repair after injury. “Our recent studies have shown that miR128 is a key regulator of heart development and that its absence allows cardiomyocyte proliferation,” Dr. Wang said. Dr. Huang presented a poster at the American Heart Association (AHA) meeting in November 2016 showing that manipulation of miR128 regulates the self-healing ability of the heart.3 This presentation was named one of the Best of Scientific Posters at the AHA meeting.
The next step, according to Dr. Wang, is a series of large animal studies using pigs. These studies are under way, and “we are already obtaining promising results,” he said.
References
1. Lescroart F, Kelly RG, Le Garrec JF, Niclas JF, Meilhac SM, Buckingham M. Clonal analysis reveals common lineage relationships between head muscles and second heart field derivatives in the mouse embryo. Development. 2010;137(19):3269-3270.
2. Harel I, Nathan E, Tirosh-Finkel L, et al. Distinct origins and genetic programs of head muscle satellite cells. Dev Cell. 2009;16:822-832.
3. Huang W. MicroRNA 128 may restore heart’s self-healing ability. Poster presented at: American Heart Association Scientific Sessions; November 11-15, 2016; Anaheim, California. Poster S1000, Session: AT.APS.P88.
Yigang Wang, MD, PD FAHA
Director, Regenerative Medicine Research
Professor, Department of Pathology and Laboratory Medicine
PHONE: (513) 558-5798
E-MAIL: yi-gang.wang@uc.edu


