Landmark Trial to Examine Cardiovascular Treatment in HIV Patients
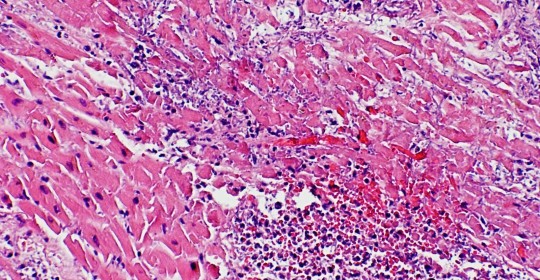
Histology of 44-year-old man with myocardial infarction and HIV demonstrating typical features of MI.
Over the course of their careers, many clinicians have seen HIV/AIDS progress from extremely high short-term mortality to chronic, long-term disease management. As HIV patients are living longer with antiretroviral therapy, it is becoming clear that the virus increases risk of cardiovascular conditions.1 The Randomized Trial to Prevent Vascular Events in HIV (REPRIEVE) study will include 6,500 participants and be conducted at approximately 100 sites. Its purpose is to test whether use of a statin medication (pitavastatin) can lower event rates for cardiovascular events such as myocardial infarctions (MIs) and strokes in this immunocompromised population.
The randomized, double-blind clinical trial is planned as an extensive, six-year study with multiple phases, recruiting HIV-infected patients between the ages of 40-75 who are not currently taking statin medication and have an American Heart Association (AHA)/American College of Cardiology (ACC) atherosclerotic risk score of ≤ 10%.2 The study is based on the current theory that the human immunodeficiency virus causes inflammation that leads to atherosclerosis, thereby increasing the risk of cardiovascular events for these patients. One of the obstacles is determining which risk factors for heart disease are genetic or lifestyle-based (i.e., smoking) and which are related directly to the virus itself and its treatment. As a result, many patients do not qualify for REPRIEVE, making the accrual phase more challenging.
Carl J. Fichtenbaum, MD, Professor of Clinical Medicine in the Division of Infectious Diseases at the University of Cincinnati Medical Center, is Vice-Chair of the REPRIEVE protocol and Chair of the Site Selection and Performance Committee. He has helped recruit the majority of the sites, and makes decisions regarding their day-to-day management, troubleshooting to ensure that each is conforming to the study protocol. The study is in its earliest stages, with more sites coming onboard each week. UC Medical Center is currently one of the top enrolling sites.
The primary endpoint of the study examines the rate of major adverse cardiovascular events (such as MIs, strokes, placement of cardiac stents, coronary artery bypass surgery, development of unstable angina and development of peripheral vascular disease) among patients in the statin group vs. the placebo group. The secondary endpoint is a measurable decrease of inflammation in the bloodstream in the two groups. There is also a mechanistic sub-study, which is examining how cardiovascular disease occurs and delving into its relationship to inflammation and the HIV virus. One question under study is whether or not the administration of a statin will lead to a decrease in the build-up of plaque in the arteries of HIV-infected patients.
Fichtenbaum sums up the study’s importance: “This may provide cardiologists with guidance on how to best manage these cardiac conditions which are being seen with greater frequency among HIV patients.” He concludes by emphasizing the uniqueness in having a mechanistic sub-study embedded within a large-scale trial of statins, seeking to discover not merely if the treatment works, but if so, how.
References: 1. Mitka M. Exploring Statins to Decrease HIV-Related Heart Disease Risk. JAMA. August 18, 2015. 314(7):657-659. 2. https://clinicaltrials.gov/ct2/show/NCT02344290?term=REPRIEVE&rank=3. Accessed October 30, 2015.3. http://www.who.int/gho/hiv/en/. Accessed November 2, 2015. 4. http://www.cdc.gov/hiv/statistics/basics/ataglance.html. Accessed November 2, 2015. 5. Freiberg MS, Chang CC, Kuller LH, Skanderson M, Lowy E, Kraemer KL et al. HIV infection and the risk of acute myocardial infarction. JAMA Intern Med. 2013 Apr 22;173(8):614-22. 6. http://www.prnewswire.com/news-releases/landmark-trial-to-explore-the-prevention-of-heart-disease-in-people-living-with-hiv-386853762.html. Accessed November 2, 2015.

Carl J. Fichtenbaum, MD
Professor of Clinical Medicine
Division of Infectious Diseases
Phone: (513) 584-6361
Email: fichtecj@ucmail.uc.edu
Connect with Carl Fichtenbaum, MD on Doximity
Web Extra:
Find a REPRIEVE site near you at: http://reprievetrial.org/collaborating-sites/
The REPRIEVE trial is being conducted at approximately 100 sites across the United States, in order to maximize convenience to clinicians who wish to refer patients to participate in this important study.

